Last Updated 1 month ago by Kenya Engineer
This article is a reminder that there has been an increasing number of failures and reports of severe corrosion in the Petrochemical Industry to structures fire protected with Cement or Epoxy paint-based materials. The aggressive corrosion attack under these materials cannot be understated.
Corrosion under these circumstances is difficult to detect by visual examination. In cases where failure is believed to be occurring can only be established by removing the fireproofing compound. This problem is not only confined to fireproofing compounds but has been found to occur beneath all major types of insulation materials for a variety of reasons.
Following are two examples of serious corrosion failures which has occurred within the Petrochemical industry.
Lightweight Cementitious Materials
In the first case, a lightweight cement mixture was applied at 50mm directly over an Inorganic Zinc Silicate primer (IZS). The original specification called for abrasive blast cleaning followed by 75microns of zinc silicate coating, followed by 50mm of lightweight cement fireproofing compound. No exterior sealing of the fireproofing was recommended at the time of installation, nor was the IZS top coated. The primary reasons for the failure was twofold, firstly the zinc coating should have been sealed, reason they have very little resistance to high alkali solutions.
Whilst Hot Dip Galvanizing is not part of this article, it is also zinc based however it has good alkali resistance and is widely used where there is a fireproofing requirements. The second reason was that the exterior of the lightweight cement material was not sealed to stop the ingress of water and soluble salts.
Corrosion Comment:
The PH factor of cement-based materials is generally 10-12.5, on the alkali side of the PH scale. When applied IZS coatings in the initial stage are quite porous; the wet cement mixture immediately penetrates and begins to attack the coating. As there is no air circulation at the interface for curing to take place; it remains wet in solution for some considerable time, this cause’s rapid consumption and deterioration of the zinc coating.
Performance under alkaline conditions is in the order of 12 – 18 months at best. There is no corrosion during this period however, when the zinc primer is consumed the alkali solution created at the interface by the cement mixture takes over and inhibits steel corrosion for as long as it remains high in alkalinity.
The second factor relates to Carbonation of the cement component caused by oxygen, together with the ingress of water and soluble salts, (marine or industrial). Cement contains calcium hydroxide which over time reacts with carbon dioxide to produce calcium carbonate. The result is reduction the PH factor back toward neutral, then onto the acidic side of the scale.
Carbonization needs to be slowed this is normally achieved by applying Anti Carbonization coatings. They are relatively inexpensive and require some maintenance every 12-15 years. Coatings used for this purpose need to be flexible and are either water or chlorinated rubber based applied to 250-300microns.
If coatings are not used for sealing purposes water will eventually penetrate, leaching alkali components to the steel surface to form the basic passive non-corrosive solution. Over time this alkali solution is converted through carbonization, which has the effect of lowering the PH factor thereby eventually losing its protective properties at which time corrosion commences.
The threshold concentration of chloride ions needed to destroy the passive solution; is estimated in the order of 700ppm at PH12. As the PH factor decreases, so does the chloride concentration necessary to cause pitting corrosion. Carbonization is at a maximum when relative humidity is above 50% and increases with increasing temperature.
The one upside to carbonization is that there is no detrimental effect to the fireproofing compound in terms of performance. The original specification should have included an alkali resistant epoxy coating to protect the IZS primer. This would have protected the primer from the alkali solution created and stopped marine or industrial chlorides penetrating to the steel substate. There is no doubt that had these two measures been taken at the time of installation the need for any refurbishment after 30 years, would have been negligible.
Epoxy Paint:
Where epoxy paint materials are used, there are still some reservations, based on fire test data there is no doubt they provide the necessary fire protection. However, they produce toxic fumes, smoke, and are one off (time) systems always needing replacement if exposed to fire.
The main concern is that in some circumstances the system, in my view appears to be fundamentally flawed in certain situations because the requirements of fire protection and corrosion of steel is difficult to combine as one. Expensive failures are all too frequent primarily due to underlining corrosion failures.
The problem described in this instance was that the anti-corrosive system beneath the fireproofing was entirely based on the use of inhibiting epoxy zinc phosphate primer applied to 125microns maximum. The applied thickness needs to be strictly controlled, as there is difficulty in gaining good adequate adhesion for the subsequent fireproofing material which was applied at 20 mm to a very smooth primer surface. There is no anchor pattern to assist adhesion although Kevlar or Fibreglass mess is used to reinforce the coating. Adhesion would be far better direct to blasted steel; unfortunately, this provides no anti-corrosive properties to protect the steel, so there is the dilemma.
Epoxy fireproofing coatings are no different than anti-corrosive epoxy paints in that they all have vapour or water transition rates, some faster than others. Anti-corrosive paints are designed to resist water, oxygen, and the ingress of soluble salts. They are commonly referred to as high molecular weight materials, in other words, the chemical structure is extremely compact and tight. Fireproofing materials are at the other end of the scale, highly filled, low molecular weight to react when exposed to fire. Microscopically they are much more open, therefore water transmission rate is much faster and higher than their anti-corrosive counterparts.
Epoxy fireproofing materials have very little anti-corrosive properties apart from behaving as a barrier to the exposed environment. Like cement-based products, they also need to be applied over anti-corrosive systems and require UV resistant topcoats. The problem with the use of epoxy primers in this instance was also twofold.
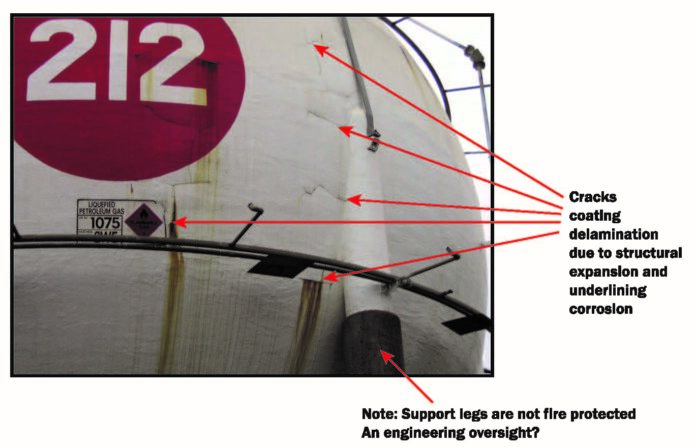
- Firstly, under normal atmospheric conditions, the expected performance of Zinc Phosphate primers without topcoats is only 12-18 months. To put this in perspective, they are not generally recommended for exposed conditions and if used must always be top coated.
- Once the zinc phosphate primer was depleted due to the ingress of water and soluble chlorides, corrosion commences creeping under the Fire Coating System. This has big implications regarding any future maintenance and cost thereof. Put simply, the primer in this instance was the wrong choice of material, a far superior system would have been a metallic zinc-based primer followed by an epoxy topcoat to 125microns.
- The second problem relates to vessel structure, sooner or later vessels come up for maintenance or are shut down for operational reasons. During these periods vessels are invariably steam cleaned, resulting in rapid steel expansion of the walls. Under these circumstances epoxy coatings expand and debond on cooling and thereafter remain detached from the steel surface.
- The other method of detachment is when they are applied to a warm operating vessel where operational shutdown occurs. In both instances the result is the creation of a void at the steel interface for water and chlorides to accumulate. Once this has occurred the performance of zinc phosphate primer is approximately 3-4 years. When the inhibiting pigments are depleted, there is nothing left at the interface to protect the steel. Unlike cement-based products, where there is an alkali solution, epoxy primers have concentrated chlorides at the interface which results in under film corrosion creep and in some instances, severe pitting.

The condition of the steel in this example, exhibited very severe corrosion due to high levels of chlorine salts. In terms of a coastal environment, the ease of removing corrosion and more significantly the water-soluble chlorides are largely dictated by the extent to which the corrosion has progressed. If severe deep pitting is encountered, the more difficult it is to remove salt contamination in any future refurbishment.
Remedial work should include high pressure water washing incorporating a chloride scavenger after conventional abrasive blasting. This operation only removes surface and visible salts but will not remove soluble salt concentrations deep within the pitted surface. Therefore, as with pinhole testing, high pressure water cleaning together with salt testing should be mandatory and part of the specification. It is when chlorides are not effectively removed, deterioration of the remedial coating occurs more readily resulting in premature failure.
Recommendations and options for hydrocarbon fire protection to mitigate corrosion of carbon steel.
- Lightweight Cement Coatings
Anti Corrosive System
Abrasive blast SA 2.5 / zinc rich epoxy primer / epoxy topcoat 300microns DFT.
Lightweight cement coating 50mm* DFT incorporating galvanized mesh.
Carbonization sealer (acrylic or chlorinated rubber) 250microns DFT
Or alternative
Hot Dip Galvanizing (EN ISO 1461,AS/NZS4680, ASTM 123 )
Lightweight cement coating 50mm* DFT
Carbonization sealer (acrylic or chlorinated rubber) 250-300microns DFT
- Epoxy Paint
Abrasive blast to white metal / zinc rich epoxy / epoxy topcoat 125microns DFT
Epoxy intumescent 8-20mm* DFT incorporating carbon fibre mesh.
Polyurethane sealer 75microns DFT (for UV resistance)
*Indicates thickness range will, depend on area / to mass ration of steel being protected.