Last Updated 1 month ago by Kenya Engineer
Breakthroughs take different forms, it is 187 years since a breakthrough in the science of corrosion prevention gave use the extraordinary benefits of long-life steel protection that we now take for granted. This article discusses a range of issues regarding the Hot Dip Galvanizing(HDG) process which involves many variables and defects that impact on the characteristics and the appearance on the product being galvanized.
HDG PROTECTIVE PRINCIPLES
Hot Dip Galvanizing dates to May 1837 when Frenchman Stanislaus Sorel filed a patent for HDG with the use of metallic zinc. To understand the significance of zinc as a protective medium it is useful to understand the protective mechanism. The function of zinc is primarily to provide the means of protecting the steel substrate, for this to occur an electrolyte (water oxygen soluble salts) needs to be present whereby an electrical current is generated between the differing metals, Zinc being more electrically active becomes sacrificial and deteriorates thereby protecting the steel substrate.
HDG is basically a mechanical handling operation where steel goes through a cleaning process prior to been dipped in to molten zinc. The process creates a metal alloy an integrated metal mixture comprising of varying degrees of Iron,Zinc,and Lead. The result is four distinct layers ( Gamma, Delta, Zeta, Eta) with an average metal weight of 58.5% Zinc,40% iron and 1.5% lead, for example, 85-micron thickness equates to a total weight of 610 grams/m2 which equals 357 grams Zinc / 244grams Iron / 9 grams lead.( Ref Fig 1)
It is not always possible to judge that the finish product created will be acceptable particularly where architectural standards are required. The prime purpose of HDG is to provide corrosion protection, which is Internationally recognised through standards such as, ISO1461,ASTM A123, and AS/NZS4680. It is important to recognize these standards only address a product description and a process method to facilitate corrosion protection and do not cover the elements of aesthetic or architectural requirements, extra specification detailing is required to cover these aspects.
HDG can be less uniform due to the inherent production process which will always highlight surface imperfections for a variety of technical reasons, many disputes arise because this is not commonly recognised by those involved in the chain of events. Where appearance is important the galvanizer should be made aware that all imperfections such as spikes, runs, lumps, and welding slag etc should be removed or avoided where necessary during the production process. Variations in steel composition can result in notable differences in appearance, even though these may be well within the standard specification required for corrosion prevention. The use of some steels can also make the creation of the desired effect less likely due to the vagaries associated with the HDG process. Where aesthetic issues are involved HDG can only deliver a level of quality within the limits of the process.
Factors Affecting HDG :
- The size and shape of the item.
- The steel chemistry.
- The surface condition.
- The metallurgy of the HDG process.
- The design of the item with respect to galvanizing.
STEEL SURFACE CONDITION

Effect: Durability unaffected. Rougher surface will produce thicker galvanized layer and give longer service life.
UNCOATED AREAS

Cause: These defects have been caused by factory applied stickers being left on the work. The adhesive cannot be removed in the pretreatment process and the steel surface does not pickle.
Remedy: Remove stickers prior to delivery/processing. Mechanically removes adhesive residues or treat with concentrated paint stripper.
ASH ON SURFACE

Cause: Ash on zinc surface trapped inside container – no access for skimming.
Remedy: Wire brush ash from surface during dressing/inspection.
ZINC SPLATTER

Cause: Moisture on surface causing molten zinc to ‘pop’ and splash droplets onto the item.
Remedy: Wire brush or sweep flakes from surface and minimise splattering by drying work as much as possible and avoiding pockets that prevent complete drainage of liquors from the surface
OXIDE LINES

Cause: Item stopped and started during withdrawal from the zinc bath because of shape or drainage conditions.
Remedy: No effect on coating other than appearance which will fade over time as the galvanized coating oxidises.
DELAMINATION

Cause: Steel high in phosphorous outside the normal phosphorous range. Steel not suitable for hot dip galvanizing – normally used for electrical laminations.
Remedy: Change steel grade.
ROUGH SURFACE
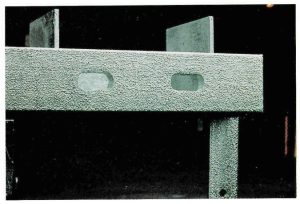
Cause: Rough surface condition of hot rolled structural section combined with high silicon content.
Remedy: Change steel grade if surface finish is an issue. Positive effect on durability because of thicker galvanized coating produced.
FISH BONING

Cause: Variations in surface chemistry on pipe feed cause minor variations in reaction rate of steel with the zinc across the surface, causing galvanized coating to vary in thickness in sharply defined zone across the surface.
Remedy: Change steel grade. This defect rarely occurs with other than imported pipe. Only effects appearance – no effect on durability.
BLEEDING

Cause: Pre-treatment chemicals penetrate unsealed joint. Moisture boils off during galvanizing leaving anhydrous crystal residues in the joint. These absorb water from the atmosphere and bleed from the joint.
Remedy: Seal weld joint where possible or leave a gap wide enough for solutions to escape and zinc penetrate. Clean up by washing. Self-limiting after crystals are hydrolysed.
SURFACE CONTAMINATION

Cause: Some types of marking paint cannot be removed in the galvanizing pre-treatment. Paint on the surface prevents the steel from pickling and the galvanized coating cannot form on the rusty surface.
Remedy: Use appropriate marking paint or chalk. Apply paint stripper to paint prior to pre-treatment. Mechanical remove paint by buffing. Avoid putting painted markings on steel prior to delivery.
DRAINAGE SPIKES
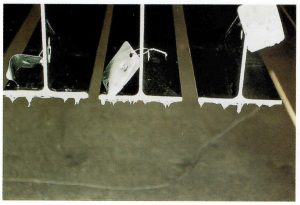
Cause: Affected surface horizontal to zinc bath on withdrawal, preventing point drainage of zinc from the surface. Difficult to eliminate from 3-D fabrications.
Remedy: Drainage spikes can be minimised by fettling the work as it emerges from the zinc bath if edges are accessible. Spikes are removed during inspection stage by buffing as a standard dressing procedure.
DROSS PIMPLES

Cause: Dross crystals from the bottom of the zinc bath picked up on surface of work and caught up in coating.
Remedy: Avoid disturbing dross layer in bath by controlling immersion depth. Appearance is affected. Positive effect on durability because of higher mass of zinc on surface
STRIATIONS

Cause: Narrow zones of higher reactivity on steel surface, usually associated with stress zones related to the manufacturing process.
Remedy: Change steel grade. Appearance is affected. No effect on durability.
DROSS

Cause: Upward-facing universal section picked up dross from bottom of zinc kettle – unable to drain out on withdrawal.
Remedy: Mechanically remove if possible or re-galvanize to melt-out dross. Costly waste for galvanizer because of the dross value. Durability unaffected because dross has similar anticorrosive properties to zinc.
RUNS

Cause: Zinc freezes on surface during draining. More likely to occur on thinner sections with large surface area that cool quickly.
Remedy: Adjust dipping angles if possible, to alter drainage pattern to more acceptable mode. Dress thick sections of coating by buffing if they interfere with the items’ application. Durability improved because of the presence of additional zinc
BLOWOUTS

Cause: Pre-treatment liquors penetrating sealed overlap areas and boiling-out during immersion in the molten zinc, causing localised surface contamination.
Remedy: Seal vent holes with temporary plugs (silicone) prior to pre-treatment. Pre-heat item prior to immersion in zinc bath to dry out overlap area as much as possible.
DISTORTION

Cause: Differential expansion and contraction of thin, flat plate during heating and cooling in the galvanizing process.
Remedy: Use thicker plate. Use ribs or corrugations to stiffen flat sections. Accept a degree of distortion (as in this case) as appropriate for application and for significant durability benefits in high abrasion environments.
TOUCH MARKS

Cause: Areas where item is resting on jigging or dipping equipment may suffer coating loss or damage because galvanized coating will bond item to support.
Remedy: Minimise contact between item and support. Damaged areas can be touched-up with appropriate zinc rich paint according to International Standard.
GREY COATINGS

Cause: Reactive (high silicon) steel in borderline zone of reactivity. Grey areas are 100% alloy layer. Shiny areas have free zinc on surface. Slower cooling will produce a higher percentage of grey coatings on this type of steel.
Remedy: Change steel grade. Cool as quickly as possible after removal from the galvanizing bath. Grey coatings are thicker and will always exceed minimum standard thickness and provide better durability as a result.
MISS

Cause: Inadequate surface preparation, usually in pickling process, and sometimes caused by local areas of heavy rust. Contamination of surface with paint or items touching during processing to prevent adequate access by pre-treatment solution or molten zinc.
Remedy: Ensure that surfaces are clean and clear prior to pre-treatment. Small missed areas can be repaired with appropriate zinc rich paint in accordance with International standards
CLOGGED HOLES
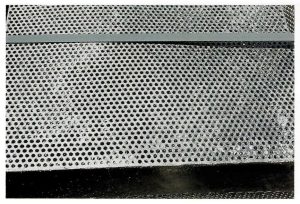
Cause: The surface tension of molten zinc is high and will not drain easily from holes under 8mm in diameter.
Remedy: Make holes as large as possible. Active fettling on bath and using vibrators on galvanizing cranes will minimise hole clogging
FLAME CUT EDGES
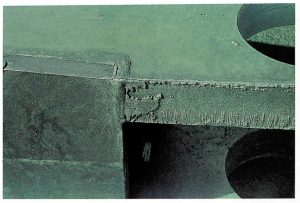
Cause: Flame cutting partly oxidises the reactive elements out of the steel surface, leaving zones of high and low reactivity.
Remedy: Grind the edges of the plate to remove heat affected surface prior to galvanizing. As long as the measurements of average coating thickness along the affected edge complies with International standards, no remedial action should be required.
WHITE RUST
Cause: Newly galvanized surfaces, exposed to fresh water (rain, dew, condensation) and not subject to good ventilation and a drying cycle will react with pure water to form zinc hydroxide.
Remedy: Passivate the item after galvanizing using a chromate quench solution. Do not stack in poorly ventilated, damp conditions. Light white rust will weather off over time in service. Severe white rust should be removed mechanically or with appropriate chemical treatment.
WELD SPATTER

Cause: Weld spatter left on surface after fabrication. Weld metal will galvanize with the parent metal.
Remedy: Weld spatter should be removed after fabrication. No effect on durability.
PEELING KIRKENDALL EFFECT


Conclusion
There are many variation that may be encountered during the HDG process some considered defects others acceptable considering the primary purpose is corrosion protection. Blowouts and bleeding or were the HDG is missing are not acceptable. Most other defects are subject to agreement between the contracting parties these have no effect on performance or durability. Many defects result in thicker HDG thereby increasing performance significantly, however an understanding of the issues involved will go a long way to ensure the process can deliver a product and standard that will satisfy architectural applications. This article is not all inclusive the intent is to provide a general insight to some of the more common variables associated with the HDG process. for further information it is recommended to consult recognized technical literature or alternatively contact your nearest HDG provider or their Industry Association.
References:
- International Galvanizing Standards :ISO1461 /ASTM123M /AS/NZS 1460
- EO Kirkendall. Zinc Diffusion in Alpha Brass Trans Aime 1947
- Professor Hido Nakaima,Osaka University Kirkendall Voids
- Architectural Galvanizing 2024 N Karakasch
- Corrosion Protection & the Environment 2024 N Karakasch
- Duplex Coating Failures with Galvanized Structures N Karakasch
- General Galvanizing Handbook UK Publication
- USA Publication TPC9 Users Guide to Galvanizing.